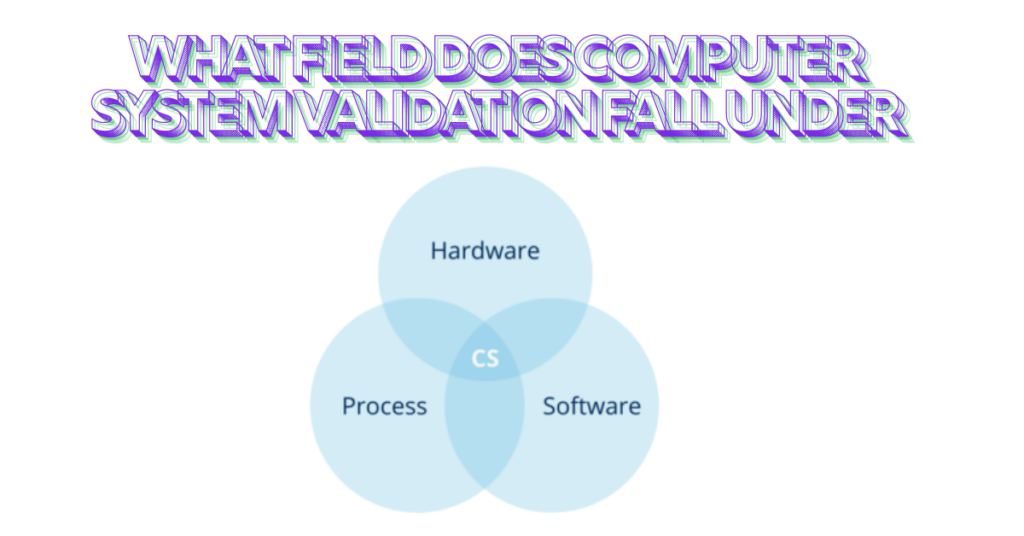
Introduction to Computer System Validation
In today’s technology-driven world, ensuring the reliability and functionality of computer systems is more critical than ever. This is especially true in sectors where human health and safety are at stake. Enter Computer System Validation (CSV)—a process that verifies whether computer systems operate as intended within regulated industries. But what field does computer system validation fall under? It spans various disciplines, intertwining quality assurance, regulatory compliance, and risk management.
As businesses increasingly rely on sophisticated software to streamline operations, understanding CSV becomes paramount. Whether it’s pharmaceutical companies developing life-saving drugs or financial institutions safeguarding sensitive data, the need for robust validation processes cannot be overstated. So let’s dive deeper into this essential component of modern business practices and explore its significance across different sectors!
The Importance of Computer System Validation
Computer System Validation (CSV) plays a vital role in ensuring the integrity and reliability of computer systems used in regulated industries. By validating these systems, organizations can confirm that they perform as intended, meeting both user requirements and regulatory standards.
The process helps reduce risks associated with software failures, data breaches, and compliance issues. This is essential for maintaining product quality and protecting patient safety in sectors like pharmaceuticals and healthcare.
Moreover, CSV fosters trust among stakeholders including clients, regulators, and partners. It demonstrates a commitment to quality assurance while enhancing operational efficiency.
Investing time and resources into CSV not only satisfies legal obligations but also positions companies for success in an increasingly digital landscape where technology drives innovation.
Key Players in the Field of CSV
The realm of Computer System Validation (CSV) is influenced by various key players. These include regulatory bodies, industry experts, and organizations that develop guidelines.
Regulatory agencies like the FDA and EMA set stringent standards for CSV practices. They ensure that systems used in critical industries adhere to safety and quality requirements.
Consultants specializing in CSV bring valuable insights into best practices. Their expertise helps companies navigate complex regulations while optimizing their validation processes.
Software developers also play a significant role. They create tools specifically designed to streamline validation efforts, making compliance more manageable for businesses.
Training providers are essential as well. They equip professionals with the necessary skills to implement effective validation protocols across different sectors.
Together, these players form a robust network that drives the evolution of computer system validation. Each contributes uniquely to maintaining high standards and ensuring operational excellence.
Industries that Require Computer System Validation
Computer system validation is crucial across various industries. The pharmaceutical sector stands out as a primary player. Here, strict regulations govern product safety and efficacy. Validated systems ensure compliance with these guidelines.
The biotechnology field also demands rigorous validation processes. Innovative therapies require precise data management to maintain quality standards throughout development.
In the medical device industry, CSV guarantees that software operates safely and effectively. This ensures patient safety and meets regulatory requirements.
Additionally, food manufacturing relies on validated systems for tracking production processes and maintaining quality control. This helps prevent contamination and ensures products meet health standards.
Clinical research organizations (CROs) utilize computer system validation extensively to manage data integrity in clinical trials—an essential aspect of obtaining reliable results.
Finance has emerged as an unexpected domain requiring CSV due to increasing reliance on technology for transaction processing and compliance reporting.
Common Standards and Regulations for CSV
Computer System Validation (CSV) is governed by several key standards and regulations that ensure compliance.
One of the most recognized frameworks is the FDA’s 21 CFR Part 11. This regulation outlines criteria for electronic records and signatures, emphasizing data integrity.
The ISO 9001 standard also plays a crucial role in CSV. It focuses on quality management systems, promoting consistent product quality across industries.
In addition, GxP guidelines—Good Practice guidelines—are essential in fields like pharmaceuticals and biotechnology. They dictate how processes should be managed to maintain compliance with regulatory standards.
Another important standard is the GAMP (Good Automated Manufacturing Practice) framework. It provides guidance on validation throughout the software lifecycle and helps streamline processes for effective validation strategies.
Understanding these regulations ensures organizations can operate within legal boundaries while maintaining high-quality output in their computer systems.
Challenges and Benefits of Implementing CSV
Implementing Computer System Validation (CSV) comes with its own set of challenges. One major hurdle is the complexity of regulatory requirements. Organizations often struggle to keep up-to-date with evolving guidelines, which can vary by industry.
Another challenge lies in resource allocation. Training staff and investing in proper tools requires time and budget, both of which can be limited. Balancing these needs against ongoing operations can create significant pressure.
Despite these challenges, there are notable benefits to implementing CSV. Enhanced data integrity stands out as a critical advantage, reducing errors that could impact product quality or compliance.
Moreover, successful validation processes lead to increased efficiency over time. Organizations experience fewer disruptions from non-compliance issues down the line, allowing for smoother operation overall.
CSV fosters trust among stakeholders—clients and regulators alike appreciate a commitment to quality control through valid practices.
Future Outlook for the Field of Computer System Validation
The future of Computer System Validation (CSV) is poised for significant evolution. As technology advances, the demand for robust validation processes will only increase. Organizations are recognizing that CSV isn’t just about compliance; it’s a crucial aspect of maintaining data integrity and quality.
Emerging technologies like artificial intelligence and machine learning are shaping how validation is approached. These innovations could streamline processes, making them more efficient while reducing human error.
Additionally, regulatory bodies are adapting to these changes. Expectations around CSV will likely become more stringent as industries strive to keep pace with technological advancements.
Data privacy concerns also drive the field forward. With stricter regulations on personal information handling, organizations must ensure their systems meet high standards of validation.
As we look ahead, collaboration between industry stakeholders will be essential in setting best practices and guidelines that resonate across multiple sectors. The landscape of CSV holds great potential for growth and innovation.
Conclusion
The field of What Field Does Computer System Validation Fall Under plays a vital role in ensuring the integrity and reliability of computer systems across various industries. As technology continues to advance, the importance of What Field Does Computer System Validation Fall Under only grows. Organizations must remain vigilant about adhering to established standards and regulations while navigating the challenges that come with implementation.
Key players, including IT professionals, quality assurance specialists, and regulatory bodies, all contribute to a robust validation process. Industries such as pharmaceuticals, healthcare, finance, and manufacturing rely heavily on What Field Does Computer System Validation Fall Under to maintain compliance and safeguard their operations.
With an evolving landscape shaped by new technologies like cloud computing and artificial intelligence, the future outlook for CSV is promising yet complex. Companies that prioritize effective validation strategies will be better positioned for success in this dynamic environment.
Understanding what field computer system validation falls under is crucial for any organization looking to leverage technology responsibly. As we continue down this path of digital transformation, staying informed about best practices in CSV will ensure both compliance and innovation thrive side by side.